Each cell within the body, despite its diverse functions, operates as an autonomous living entity and must effectively manage the resources it possesses. Mammalian cells employ various substrates, such as glucose and fatty acids, to derive energy through oxidation. This energy is then allocated to a range of tasks, including protein synthesis or the transmission of nerve signals. However, within a healthy organism, specialized cells handle energy distinctively. For instance, adipocytes specialize in storing energy substrates for times of scarcity, whereas neurons lack storage capacity and therefore require a continuous energy supply due to their substantial consumption rate. Besides the significant diversity in the functioning of individual cell types, the communication between different cells in the mammalian organism is also fascinating.
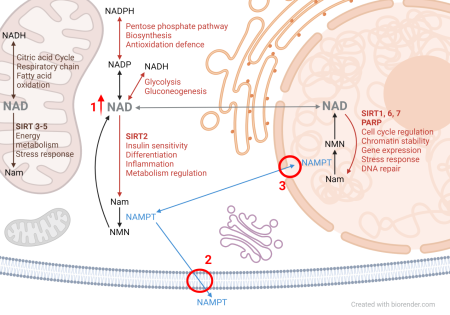
The key molecule in the energy metabolism of all cells is nicotinamide adenine dinucleotide (NAD). The diagram simplifies the main processes that utilize NAD. NAD serves to transfer electrons from redox catabolic reactions to the respiratory chain for energy acquisition. The phosphorylated form of NAD (NADP) is indispensable for biosynthetic pathways. Redox reactions are characterized by converting NAD and NADP between their oxidized and reduced forms, without affecting the overall quantity of these molecules.
However, some reactions consume NAD. The most significant enzymes catalyzing these reactions are the signaling proteins from the sirtuin family (SIRT). Sirtuins deacetylate proteins by transferring the acetyl group onto NAD, releasing nicotinamide (Nam), thereby regulating the function and activity of target proteins, including histones, transcription factors, and enzymes. Sirtuins primarily act in the nucleus, where they regulate gene expression and chromatin stability, but also in the cytosol and mitochondria, where they participate in metabolism regulation.
Another group of enzymes that consume NAD are mono- and poly-ADP-ribosyltransferases (PARP), which covalently modify proteins with an ADP-ribose residue, releasing nicotinamide. This process regulates DNA repair, among other functions. Both redox and non-redox reactions utilize the same pool of NAD, which can become depleted during high sirtuin and PARP activity, leading to cellular energy metabolism collapse and cell death.
NAD replenishment in mammals primarily occurs through the nicotinamide salvage pathway. Initially, Nam combines with phosphoribosylpyrophosphate (PRPP) to form nicotinamide mononucleotide (NMN) via the enzyme nicotinamide phosphoribosyltransferase (NAMPT). NMN then combines with adenosine triphosphate (ATP) to form NAD. This pathway occurs in both the nucleus and cytosol, although it's not precisely understood if it also takes place in mitochondria or how NAD is transported into mammalian cell mitochondria. NAMPT activity determines the rate of the entire biosynthetic pathway and, consequently, the cellular NAD level.
We are a small team of scientists focusing on the study of NAD metabolism, particularly the enzyme NAMPT. We are working on these specific projects:
- The impact of increased NAD levels on the metabolism of healthy and damaged cells. With age, chronic diseases tend to increase while NAD levels decline. By using various precursors or increasing the amount of NAMPT, it may be possible to raise NAD levels. Can boosting NAD levels be used as supportive therapy for chronic diseases such as type 2 diabetes? We are interested in understanding how increasing NAD levels will affect metabolism, gene expression, and the secretory profile of various cell types, with the aim of potentially using NAD precursors in medicine.
- NAMPT secretion. An extracellular form of NAMPT has been described, but it's not clear how this enzyme is secreted from cells or what its role is in extracellular space. We are working to clarify these uncertainties.
- Nuclear transport of NAMPT. NAMPT functions in both the cytosol and the nucleus, and it is present in both compartments. However, it's not clear how NAMPT is transported between the cytosol and the nucleus or how this process is regulated. Our work, which has shown how the transport of NAMPT into the nucleus is related to the cell cycle, has been featured on the cover of the prestigious Journal of Biological Chemistry, and we plan to continue investigating this topic.
Cooperation
- Energy Homeostasis Section, Diabetes, Endocrinology, & Obesity Branch, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, USA.
- Laboratory of Translational and Experimental Diabetology and Obesitology, Institute for Clinical and Experimental Medicine, PragueL
- Laboratory of Integrative Structural Biology, Institute of Experimental Botany of the Czech Academy of Sciences, v. v. i.
- Institute Of Physiology 0f The Czech Academy of Sciences, v. v. i.
- Czech Society for Atherosclerosis
Selected publications:
- Škop V, Guo J, Liu N, Xiao C, Hall KD, Gavrilova O, Reitman ML: The metabolic cost of physical activity in mice using a physiology-based model of energy expenditure. Molecular metabolism. 2023, 71: 101699.
- Vacurova E, Trnovska J, Svoboda P, Skop V, Novosadova V, Reguera DP, Petrezselyová S, Piavaux B, Endaya B, Spoutil F, Zudova D, Stursa J, Melcova M, Bielcikova Z, Werner L, Prochazka J, Sedlacek R, Huttl M, Hubackova SS, Haluzik M, Neuzil J: Mitochondrially targeted tamoxifen alleviates markers of obesity and type 2 diabetes mellitus in mice. Nature communications 2022, 13(1):1866.
- Škop V, Guo J, Liu N, Xiao C, Hall KD, Gavrilova O, Reitman ML: Mouse thermoregulation: introducing the concept of the thermoneutral point. Cell reports 2020, 31(2):107501.
- Svoboda P, Krizova E, Sestakova S, Vapenkova K, Knejzlik Z, Rimpelova S, Rayova D, Volfova N, Krizova I, Rumlova M, Sykora D, Kizek R, Haluzik M, Zidek V, Zidkova J, Skop V.: Nuclear transport of nicotinamide phosphoribosyltransferase is cell cycledependent in mammalian cells, and its inhibition slows cell growth. Journal of Biological Chemistry 2019, 294(22):8676-8689.
- Svoboda P, Křížová E, Čeňková K, Vápenková K, Zídková J, Zídek V, Škop V: Visfatin is actively secreted in vitro from U-937 macrophages, but only passively released from 3T3-L1 adipocytes and HepG2 hepatocytes. Physiological Research 2017, 66(4):709-714.
- Skop V, Cahova M, Dankova H, Papackova Z, Palenickova E, Svoboda P, Zidkova J, Kazdova L: Autophagy inhibition in early but not in later stages prevents 3T3-L1 differentiation: Effect on mitochondrial remodeling. Differentiation 2014, 87(5):220-229.

